Science
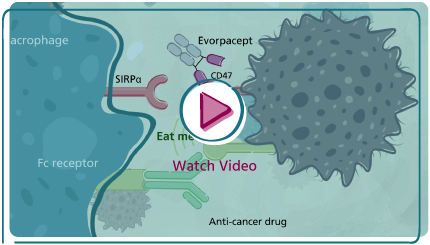
Cancer cells leverage CD47, a cell surface protein, as a “don’t eat me” signal to evade macrophage phagocytosis or as a “don’t activate T-cell” signal that prevents activation of T-cells by dendritic cells. Our company is developing a next-generation checkpoint inhibitor designed to have a high affinity for CD47 and to avoid the limitations caused by hematologic toxicities inherent in other CD47 blocking approaches.
Evorpacept is a highly differentiated
Evorpacept Development
We are pursuing a dual development path based on two differentiated mechanisms of action while at the same time evaluating the contribution of chemotherapy on top of them. The potential mechanism of action of evorpacept is dependent on the class of combination agent.
The distinct mechanism of actions and examples of the combinations are:
- Anti-cancer antibodies (the “don’t eat me” signal): Combining with anti-cancer targeted antibodies with an active Fc domain, where evorpacept enables the Fc-mediated antibody dependent phagocytosis that is impaired by the expression of CD47 on cancer cells.
An example of this mechanism is in our clinical trial, ASPEN-06, a randomized study evaluating the contribution of evorpacept to Herceptin® (traztuzumab) plus standard of care versus standard of care (CYRAMZA® + paclitaxel) in second line or later HER2-positive gastric/gastroesophageal junction cancer. We believe this mechanism is clinically validated by our Phase 1 data in combination with Herceptin in gastric cancer and in combination with rituximab in non-Hodgkin lymphoma where in both settings we saw improvement on overall response rate with respect to historical data. Positive data in ASPEN-06 will represent proof of concept for this mechanism and could lead to combinations with other anti-cancer antibodies. We have already initiated multiple clinical studies based on this mechanism (see Company pipeline) to rapidly expand the use of evorpacept in combination with this class of agents in multiple indications. This same mechanism of action also applies to antibody-drug conjugates which we are currently developing in two separate Phase 1 trials: one in combination with PADCEV® (enfortumab vedotin-ejfv) in patients with urothelial cancer, and the other in combination with ENHERTU® (fam-trastuzumab deruxtecan-nxki) in patients with unresectable or metastatic HER2-positive and HER2-low breast cancer. - PD-1/PD-L1 immune checkpoint inhibitors (the “don’t activate T-cell” signal): Combining with PD-1/PD-L1 checkpoint inhibitor, where evorpacept activates dendritic cells that are constitutively inhibited by the CD47/ SIRP alpha pathway.
Activated dendritic cells present neoantigens to T-cells that once activated will kill cancer cells when the PD-1/PD-L1 inhibitory interaction is blocked by T-cell checkpoint inhibitors. The combination with a PD-1/PD-L1 checkpoint inhibitor allows the maximum activity of these newly activated T-cells. Examples of this mechanism are our clinical trials, ASPEN-03 and ASPEN-04, two randomized studies in combination with KEYTRUDA® (pembrolizumab), without or with chemotherapy respectively, comparing to the same treatments without evorpacept, in first line head and neck squamous cell carcinoma. This mechanism is validated by our Phase 1 data in the same indications and combinations where we saw an improvement in overall survival at 12 months with respect to historical data.
Based on our preliminary clinical results to date in multiple oncology indications showing encouraging anti-tumor activity and tolerability and our clinical development plans, our strategy is to pursue evorpacept as a potentially critical component of future oncology combination treatments.
Evorpacept: Designed to be Best-in-Class CD47 Checkpoint Inhibitor
Publications
- A Phase 1a Study of Evorpacept plus Enfortumab Vedotin in Patients with Locally Advanced or Metastatic Urothelial Carcinoma View poster (ASCO 2024)
- A Phase 2 Study of Evorpacept, Cetuximab, and Pembrolizumab in Patients with Refractory Microsatellite Stable Metastatic Colorectal Cancer View poster (ASCO 2024)
- A Phase 1 Investigator-Initiated Trial of Evorpacept (ALX148), Lenalidomide and Rituximab for Patients with Relapsed or Refractory B-Cell non-Hodgkin lymphoma View presentation (AACR 2024)
- A Phase 1 Study of Azacitidine in Combination with Evorpacept for Higher-Risk Myelodysplastic Syndrome (MDS); ASPEN-02 View poster (AACR 2024)
- Evorpacept, a CD47-Blocking Myeloid Checkpoint Inhibitor, in Combination with Azacitidine and Venetoclax in Patients with Acute Myeloid Leukemia (ASPEN-05): Results from Phase 1a Dose Escalation Part View poster (ASH 2022)
- A Phase 2 Study of Evorpacept (ALX148) in Combination with Pembrolizumab and Chemotherapy in Patients with Advanced Head and Neck Squamous Cell Carcinoma (HNSCC); ASPEN-04 View poster (SITC 2022)
- A Phase 2 Study of Evorpacept (ALX148) in Combination with Pembrolizumab in Patients with Advanced Head and Neck Squamous Cell Carcinoma (HNSCC); ASPEN-03 View poster (SITC 2022)
- Evorpacept (ALX148), a CD47-Blocking Myeloid Checkpoint Inhibitor, in Combination with Azacitidine: A Phase 1/2 Study in Patients with Myelodysplastic Syndrome
(ASPEN-02) View poster (ASH 2021) - Evorpacept Alone and in Combination with Pembrolizumab or Trastuzumab in Patients with Advanced Solid Tumors
(ASPEN-01) : a First-in-Human, Open-Label, Multicentre, Phase 1 Dose-Escalation and Dose-Expansion Study View article (The Lancet Oncology 2021) - A Phase 2 Study of Evorpacept (ALX148) in Combination with Pembrolizumab and Chemotherapy in Patients with Advanced Head and Neck Squamous Cell Carcinoma (HNSCC);
ASPEN-04 View poster (SITC 2021) - A Phase 2 Study of Evorpacept (ALX148) in Combination with Pembrolizumab in Patients with Advanced Head and Neck Squamous Cell Carcinoma (HNSCC);
ASPEN-03 View poster (SITC 2021) - ALTA-002, a SIRPα-Directed TLR9 Agonist Antibody Conjugate, Activates Myeloid Cells and Promotes Anti-Tumor Immunity View poster (SITC 2021)
- Evorpacept (ALX148), a CD47 Myeloid Checkpoint Inhibitor, in Patients with Head and Neck Squamous Cell Carcinoma (HNSCC) and with Gastric/Gastroesophageal Cancer (GC); ASPEN-01 View poster (SITC 2021)
- ASPEN-01: A Phase 1 Study of ALX148, a CD47 Blocker, in Combination with Trastuzumab, Ramucirumab, and Paclitaxel in Patients with 2nd Line HER2-Positive Advanced Gastric or Gastroesophageal Cancer View poster (ESMO-GI 2021)
- ALX148, a CD47 Blocker, in Combination with Rituximab in Patients with Relapsed/Refractory (R/R) Non-Hodgkin Lymphoma; ASPEN-01 View slides (ASH 2020)
- ALX148 Enhances the Depth and Durability of Response to Multiple AML Therapies View slides (ASH 2020)
- Targeting the Myeloid Checkpoint Receptor SIRPα Potentiates Innate and Adaptive Immune Responses to Promote Anti‑Tumor Activity View article (JHO 2020)
- ALX148, a CD47 Blocker, in Combination with Standard Chemotherapy and Antibody Regimens in Patients with Gastric/Gastroesophageal Junction (GC) Cancer and Head and Neck Squamous Cell Carcinoma (HNSCC); ASPEN-01 View poster (SITC 2020)
- Assessing and Mitigating the Interference of ALX148, a Novel CD47 Blocking Agent, in Pretransfusion Compatibility Testing View article (Transfusion 2020)
- ALX148, a CD47 Blocker, in Combination with Rituximab in Patients with Relapsed/Refractory (R/R) Non-Hodgkin Lymphoma View poster (EHA 2020)
- A Phase 1 Study of ALX148, a CD47 Blocker, in Combination with Standard Anti Cancer Antibodies and Chemotherapy Regimens in Patients with Advanced Malignancy View poster (ASCO 2020)
- A Phase 1 Study of ALX148, a CD47 Blocker, in Combination with Rituximab in Patients with Non-Hodgkin Lymphoma View poster (ASH 2019)
- Pharmacodynamic Biomarker Characterization of ALX148, a CD47 Blocker, in Combination with Established Anticancer Antibodies in Patients with Advanced Malignancy View poster (SITC 2019)
- Discovery of High Affinity, Pan-Allelic, and Pan-Mammalian Reactive Antibodies Against the Myeloid Checkpoint Receptor SIRPα Read article (mAbs 2019)
- A Phase 1 Study of ALX148, a CD47 Blocker, in Combination with Established Anticancer Antibodies in Patients with Advanced Malignancy View poster (ASCO 2019)
- Antibodies to SIRPα Enhance Innate and Adaptive Immune Responses to Promote Anti-Tumor Activity View poster (AACR 2019)
- Discovery of Monoclonal Antibodies Targeting Myeloid Checkpoint SIRPα to Enhance Anti-Tumor Immunity View poster (Keystone 2019)
- ALX148 Blocks CD47 and Enhances Innate and Adaptive Antitumor Immunity with a Favorable Safety Profile Read article (PLOS ONE 2018)
- A Phase 1 Study of ALX148: CD47 Blockade in Combination with Anti-Cancer Antibodies to Bridge Innate and Adaptive Immune Responses for Advanced Malignancy View poster (SITC 2018)
- Pharmacokinetic and Pharmacodynamic Characterization of ALX148, a CD47 Blocker, in Patients with Advanced Malignancy and Non-Hodgkin Lymphoma View poster (SITC 2018)
- Oral Presentation: A Phase 1 Study of ALX148: CD47 Blockade in Combination with Anti-Cancer Antibodies to Bridge Innate and Adaptive Immune Responses for Advanced Malignancy View slides (SITC 2018)
- A Phase 1 Study of ALX148, a CD47 Blocker, Alone and in Combination with Established Anti-Cancer Antibodies in Patients with Advanced Malignancy and Non Hodgkin Lymphoma View poster (ASCO 2018)
- Oral Presentation: ALX148 is a High Affinity SIRPα Fusion Protein that Blocks CD47, Enhances the Activity of Anti-Cancer Antibodies and Checkpoint Inhibitors, and Has a Favorable Safety Profile in Preclinical Models View slides (ASH 2017)
- ALX148 is a High Affinity SIRPα Fusion Protein that Blocks CD47, Enhances the Activity of Anti-Cancer Antibodies and Checkpoint Inhibitors, and Has a Favorable Safety Profile in Preclinical Models View abstract (Blood 2017)
- A First-in-Human Study of ALX148: CD47 Blockade to Enhance Innate and Adaptive Immunity for Advanced Solid Tumor Malignancy and Non-Hodgkin Lymphoma View poster (SITC 2017)