Evorpacept
A Cornerstone of Future Oncology Therapy
ALX Oncology's lead therapeutic candidate, evorpacept, is a novel, highly differentiated CD47-targeted investigational therapy.
Learn more by viewing our Publications.
By blocking the “don’t eat me” signal transmitted by CD47 on the surface of cancer cells, evorpacept uniquely reveals these cells to the innate immune system, helping to unleash the full power of the body’s natural defenses against cancer and potentially boosting responses to other important cancer therapies.
In clinical studies across a wide spectrum of tumor types in more than 700 patients to date, evorpacept has demonstrated the potential to serve as a cornerstone therapy upon which the future of immuno-oncology can be built.
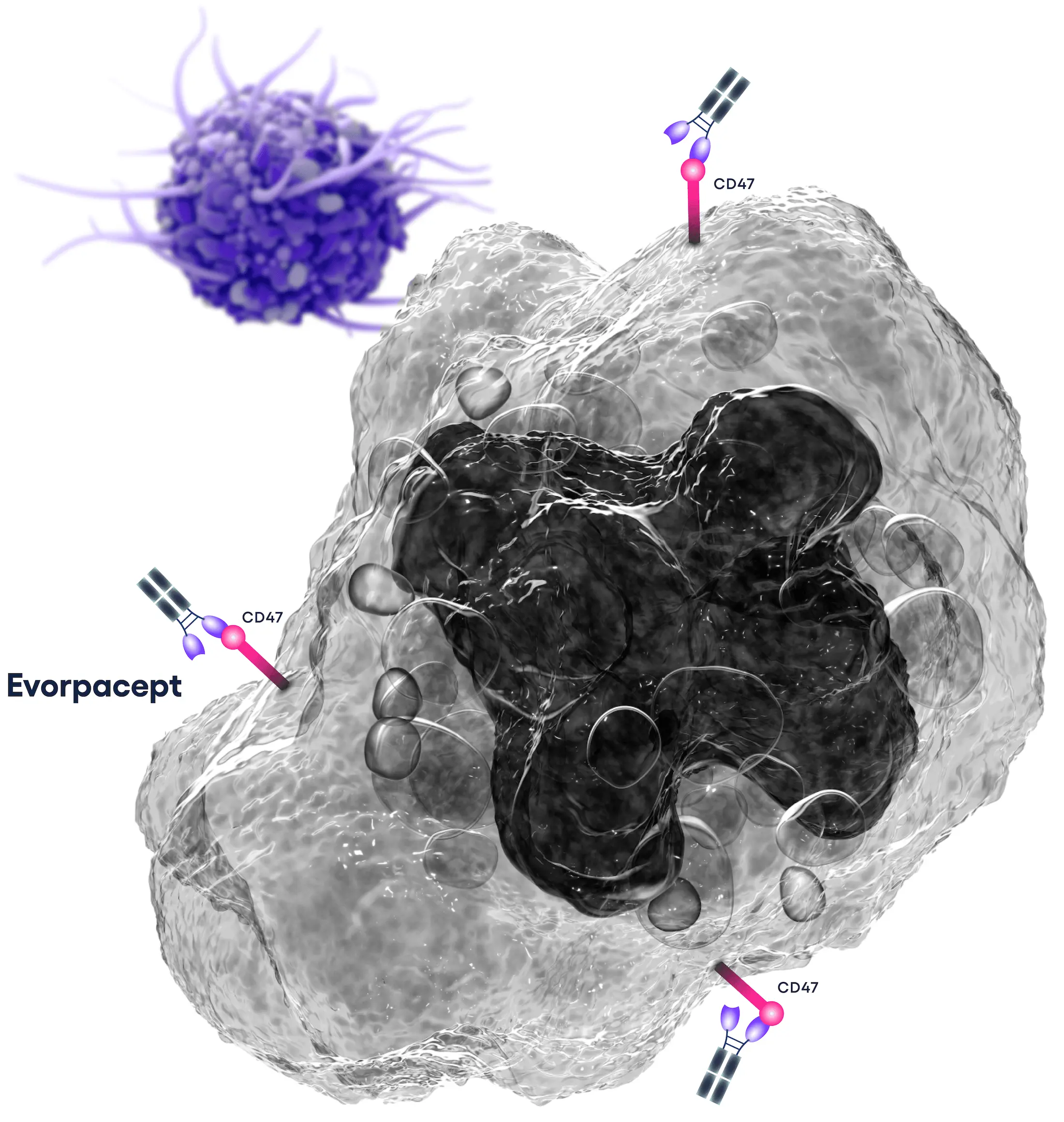
What Is CD47?
CD47 is a protein found on almost all human cells, including red blood cells. It binds to the SIRPα receptor on certain immune system cells (macrophages and dendritic cells), sending a "don't eat me" signal that inhibits absorption by these cells. This action helps protect healthy cells by signaling immune cells, “I belong here – don’t eat me!” However, many tumor cells overexpress CD47, allowing them to evade immune detection and avoid destruction by macrophages.
Previous CD47-Blocking Approaches Fell Short
Other CD47-blocking approaches combine a CD47-binding domain with an active Fc domain, part of an antibody structure that binds to Fc receptors on immune cells to help generate an immune response. These molecules bind to CD47 on the surface of cancer cells and, on their active Fc end, to Fcγ receptors on macrophages and other immune system cells. Ultimately, this action can generate an indiscriminate “eat me” signal against normal cells that is associated with significant side effects and dose-limiting toxicities. In addition, the low CD47 binding affinity of these molecules, combined with dosing limitations, can result in some CD47 receptors on cancer cells remaining unbound and free to exercise their “don’t eat me” signal. This can limit their efficacy in the combination setting.
Greater Benefit, Broader Impact
Research has demonstrated that evorpacept has the potential to enhance the therapeutic activity of many of the most important cancer therapies available today.
This boosting mechanism could expand the impact of immunotherapy and improve outcomes for many patients.
By blocking the "don't eat me" signal transmitted by CD47 on the surface of cancer cells, evorpacept is designed to uniquely reveal these cells to the innate immune system, allowing the body's natural defenses against cancer to engage, and potentially boost responses to other important cancer therapies.
Evorpacept may contribute an additional, differentiated immuno-oncology mechanism to anti-cancer antibodies, creating the possibility of a more robust immune response to the disease. These therapies stimulate the immune system to attack cancer, and evorpacept may play a critical role by making cancer cells more visible to this attack.
Evorpacept + Anti-Cancer Antibodies
Validated Mechanism
Evorpacept is a highly differentiated agent that, due to its proprietary design and the broad expression of CD47 across various cancers, holds the potential to be a significant advancement in cancer care.

Extensive preclinical research utilizing solid tumor and hematologic models indicated that evorpacept binds and blocks CD47 with high affinity. In animal models, this blockade boosted immune activity and improved tumor cell recognition and clearance.
Evorpacept enhanced anti-tumor monoclonal antibody (mAB) and checkpoint inhibitor activity by activating dendritic cells, stimulating macrophage activity, enhancing T-cell activity through cross-priming, and inhibiting immune-suppressive cells that can dampen anti-tumor responses in pre-clinical models. These data supported the clinical approach for evorpacept, which has now exhibited anti-tumor activity in a randomized Phase 2 ASPEN-06 clinical trial.
Based on the demonstrated potential of evorpacept, we are advancing a focused clinical program evaluating this novel CD47-blocking therapy in multiple cancer indications.
Explore Our Pipeline